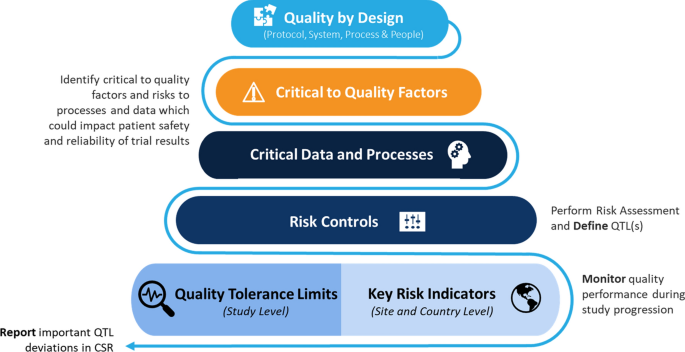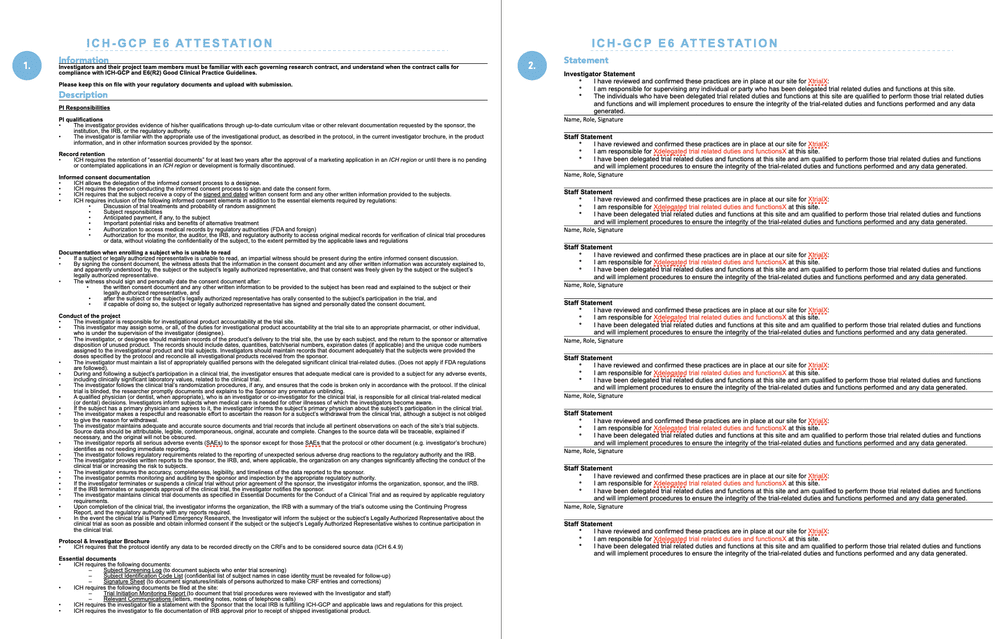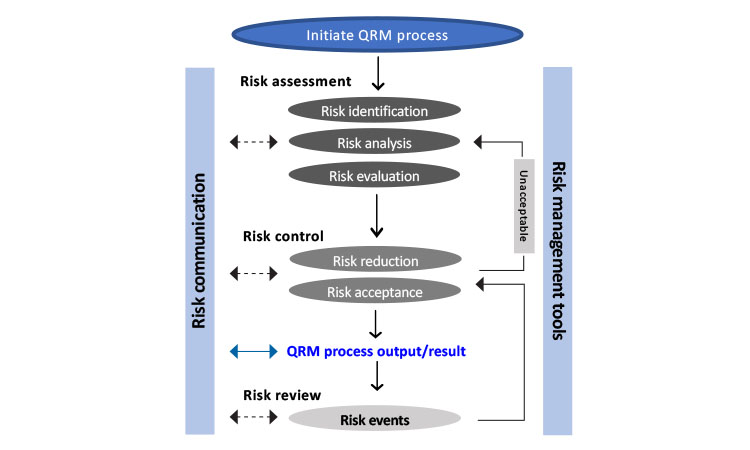
Clinical Trial Management Adaptation to ICH E6 (R2): Good Clinical Practice | Pharmaceutical Engineering
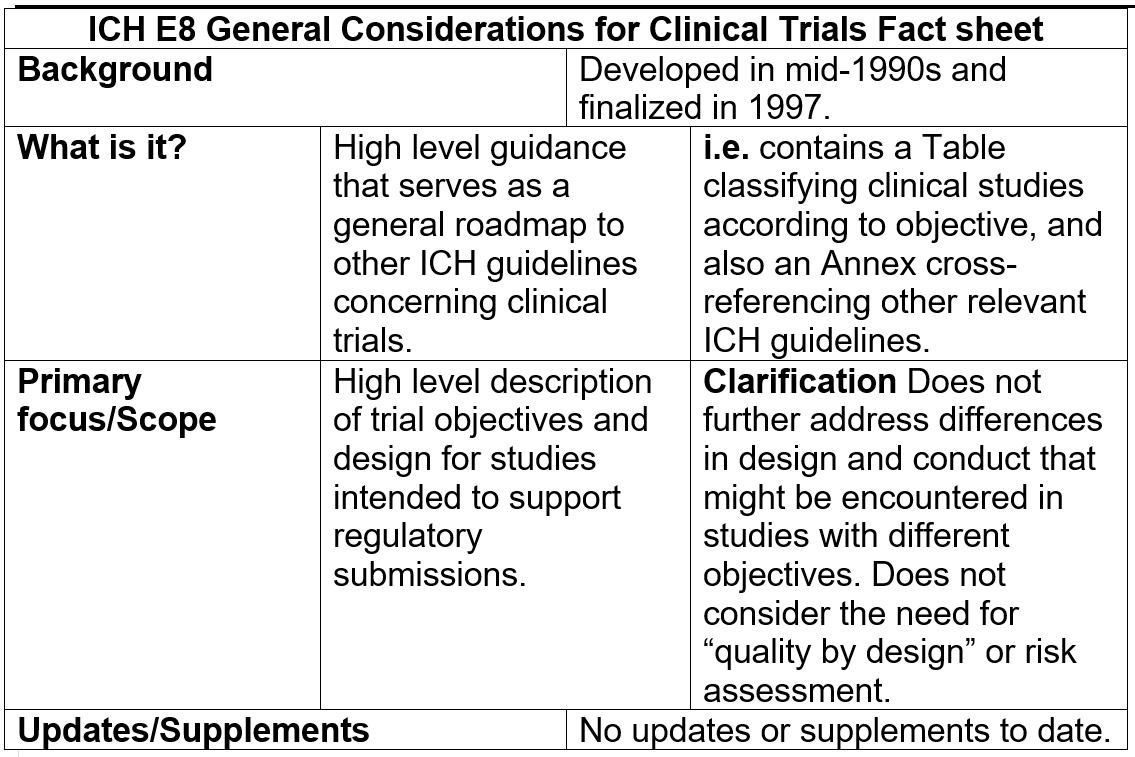
GCP Renovation” for the Modernization of ICH E8 and Subsequent Renovation of ICH E6! Series Part 3/4 — Clinical Pathways

The Impact of ICH GCP E6 Guideline R2 Revisions on Sponsors, Sites, Contract Research Organizations and Vendors | Pharmaceutical Outsourcing - The Journal of Pharmaceutical & Biopharmaceutical Contract Services

Final ICH GCP E6 R2 Addendum: Overview of Changes Impacting Sponsors/CROs/Clinical Investigator/Site - YouTube