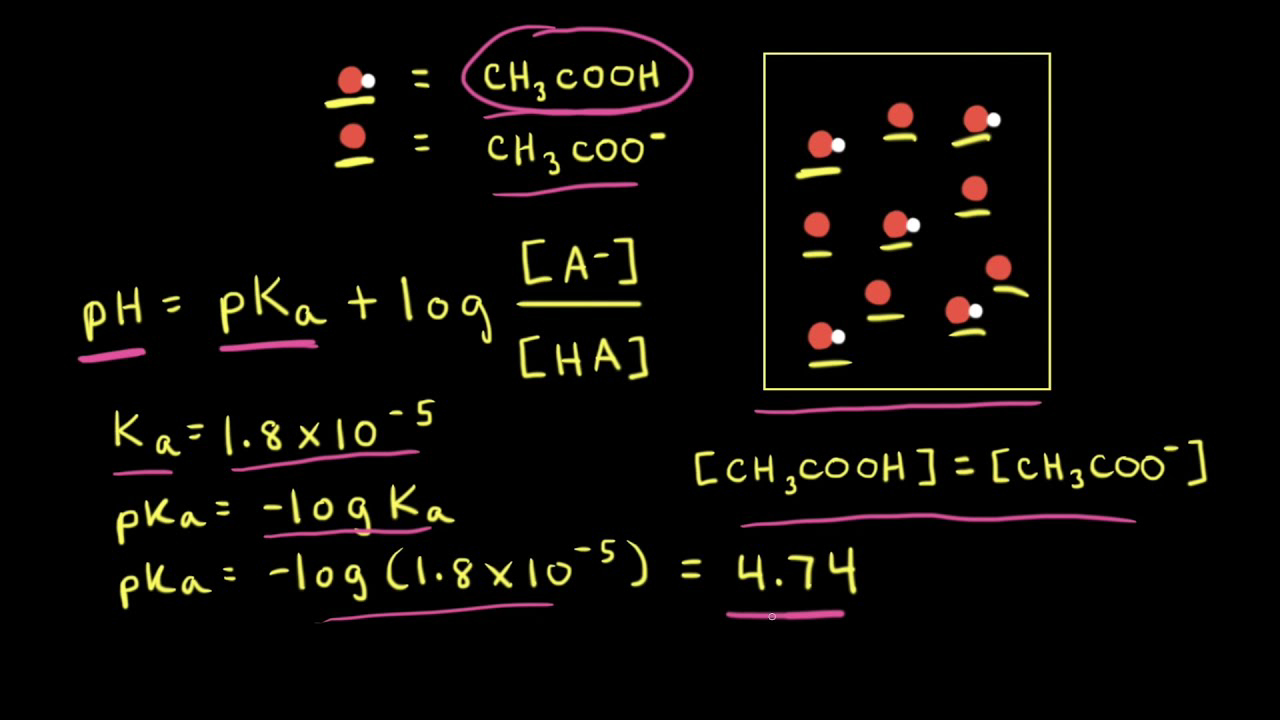
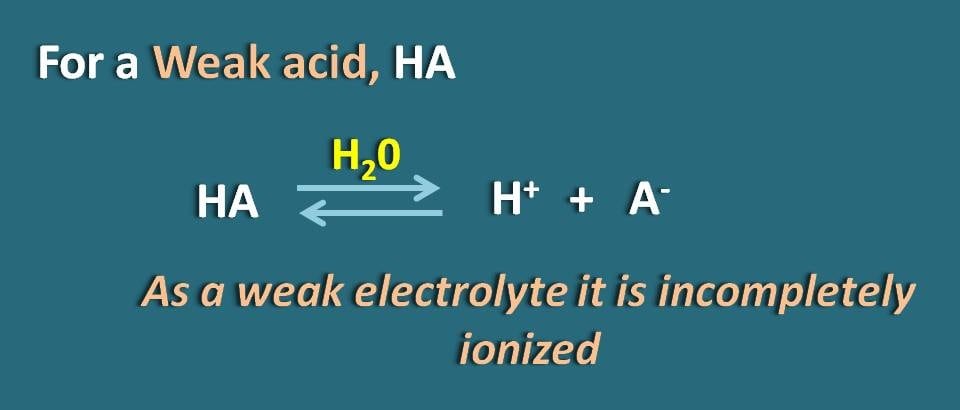
![OneClass: "Calculate the corresponding [H3O+], compute the concentration of A- and HA, and calculate ... OneClass: "Calculate the corresponding [H3O+], compute the concentration of A- and HA, and calculate ...](https://prealliance-textbook-qa.oneclass.com/qa_images/homework_help/question/qa_images/116/11663947.jpeg)
OneClass: "Calculate the corresponding [H3O+], compute the concentration of A- and HA, and calculate ...
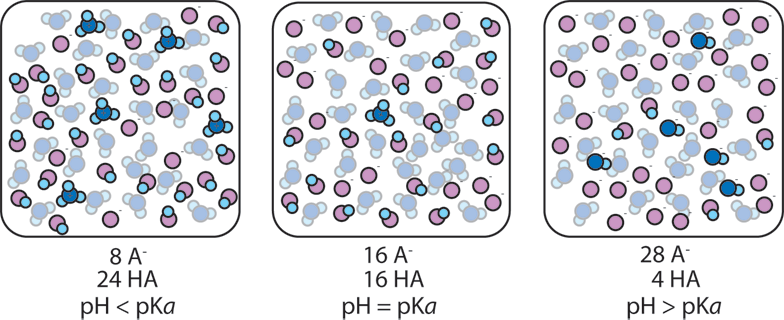
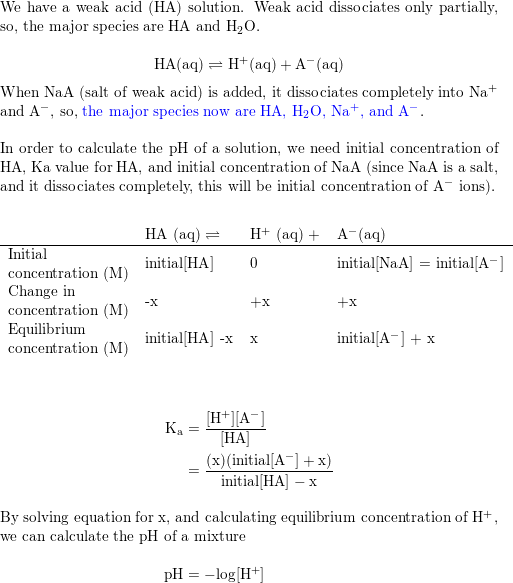
![SOLVED: What is the [A-] / [HA] ratio necessary to make a buffer solution with a pH of 4.14? Ka = 1.85 x 10-5 for HA. Show formula and calculation with units. SOLVED: What is the [A-] / [HA] ratio necessary to make a buffer solution with a pH of 4.14? Ka = 1.85 x 10-5 for HA. Show formula and calculation with units.](https://cdn.numerade.com/ask_previews/a61d22d0-b9a8-4b14-87ab-a744b7801b70_large.jpg)
SOLVED: What is the [A-] / [HA] ratio necessary to make a buffer solution with a pH of 4.14? Ka = 1.85 x 10-5 for HA. Show formula and calculation with units.
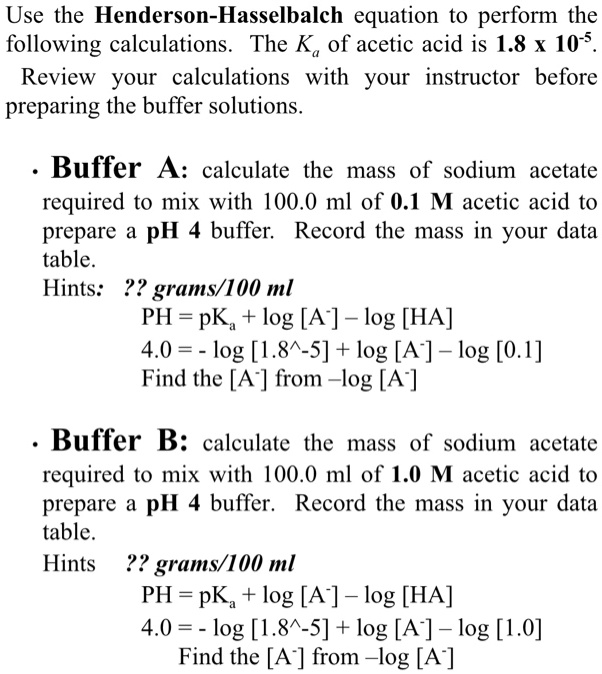
SOLVED: Use the Henderson-Hasselbalch equation to perform the following calculations. The Ka of acetic acid is 1.8 10-s Review your calculations with your instructor before preparing the buffer solutions Buffer A: calculate

Calculation of additional processor profit ($ ha -1 ) for a field in a... | Download Scientific Diagram