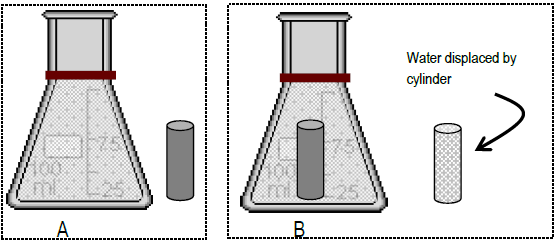
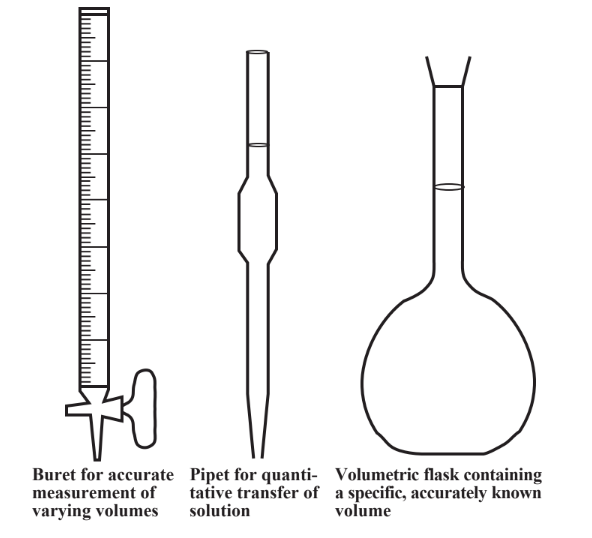
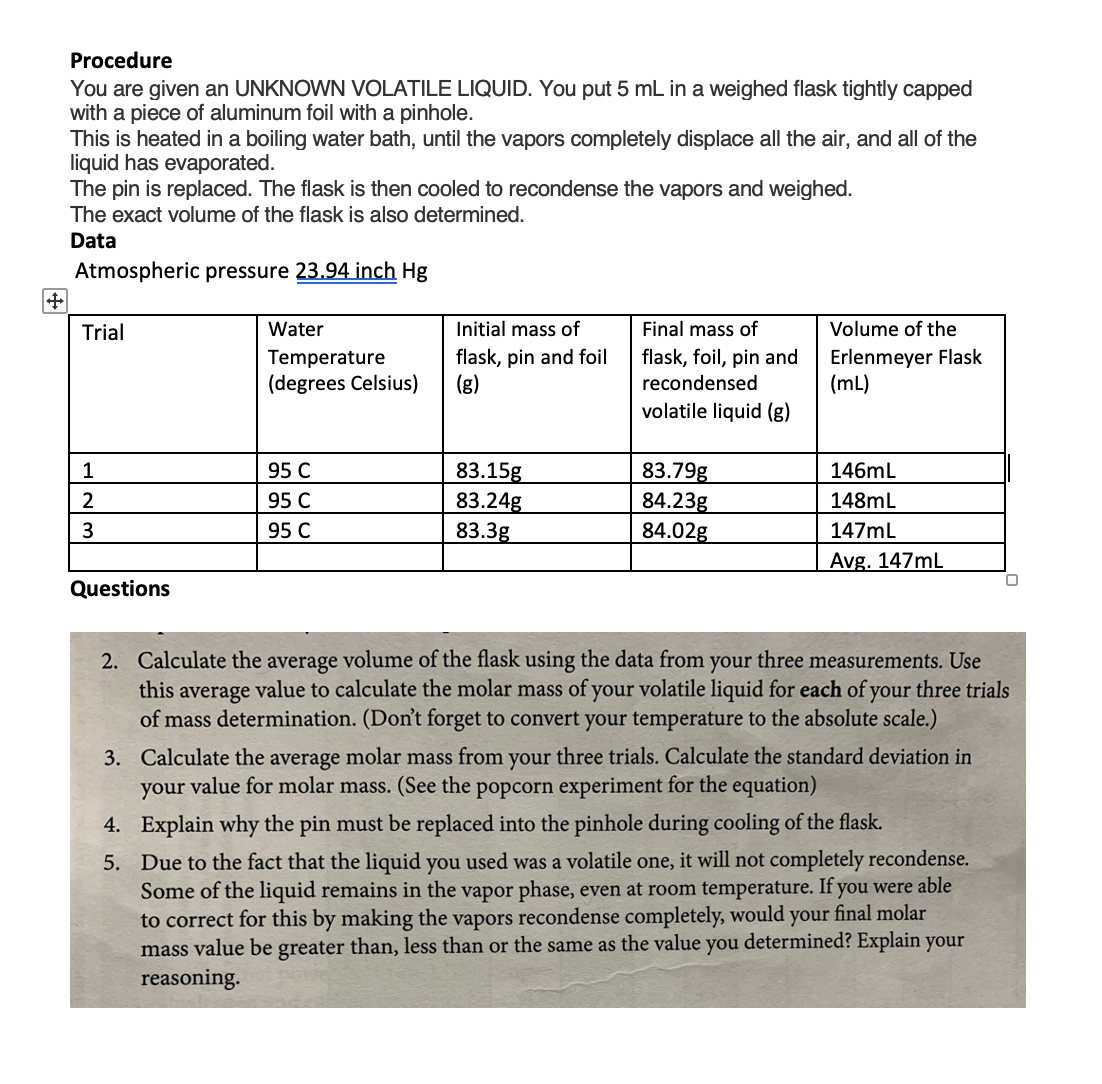
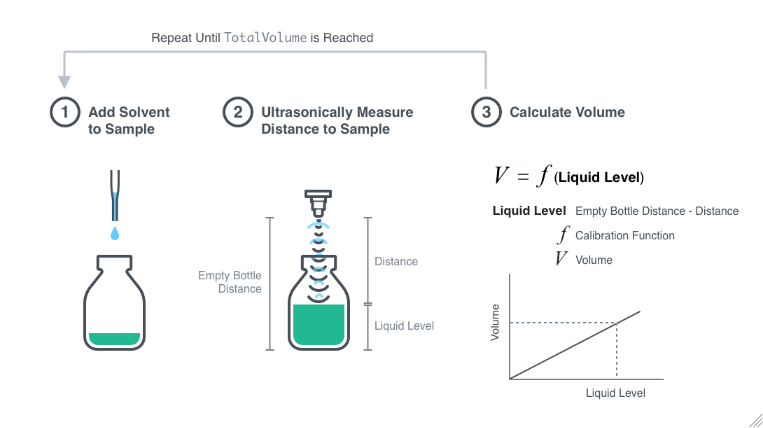
The volume of vapor in a flask can be determined by two methods: either by determining the mass of water required to just fill the flask completely or by using a graduated
Two flasks of equal volume have been joined by narrow tube of negligible volume .Initially both flasks are at 300 Kelvin containing 0.60mol of oxygen gas at 0.5 ATM pressure . One

Two flask of equal volume are connected by a narrow tube (of negligible volume) are at `27^(@)C` and - YouTube

SOLVED:An empty Erlenmeyer flask weighs 241.3 g. When filled with water (d=1.00 g / cm^3), the flask and its contents weigh 489.1 g . (a) What is the flask's volume? (b) How

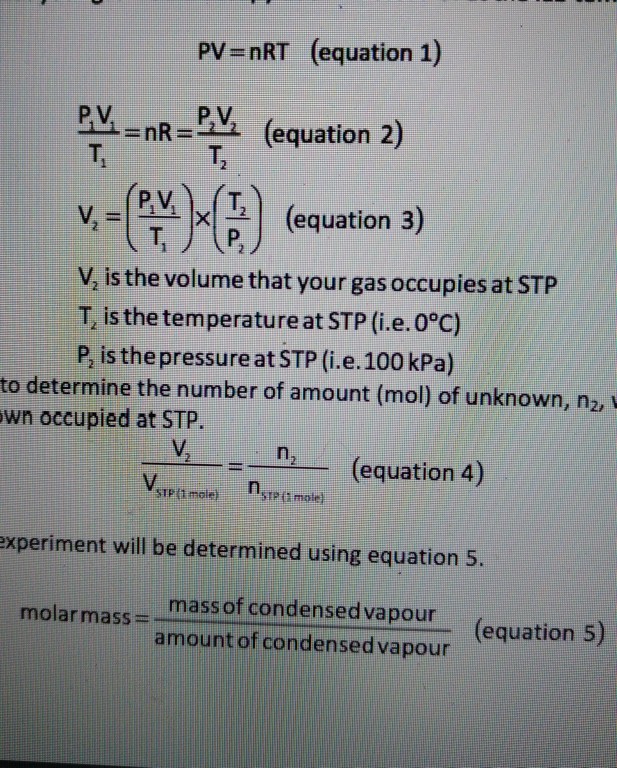
SOLVED:To find the volume of a flask, the flask is evacuated so it contains no gas. Next, 4.4 g CO2 is introduced into the flask. On warming to 27^∘ C, the gas
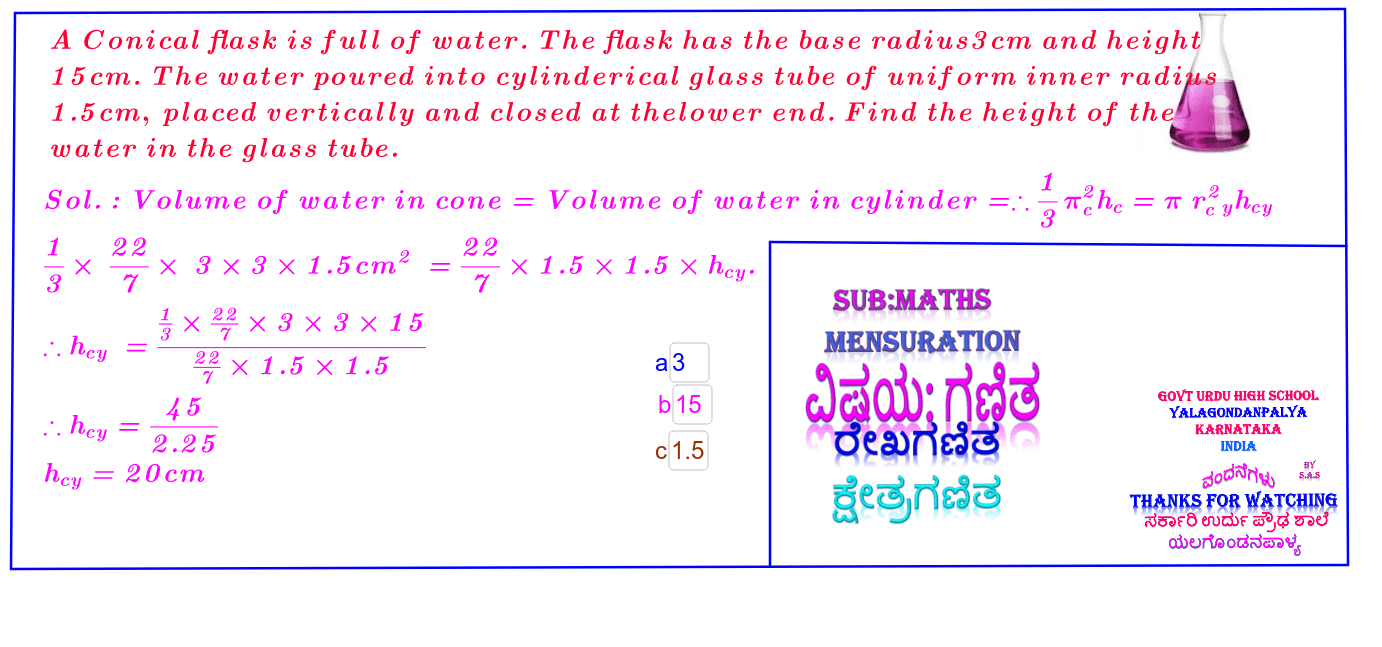
What is the volume of a conical flask which is 13 cm in height, with base radius of 6 cm, and an upper radius of 2 cm? - Quora

SOLVED:Finding the volume of a flask. A student obtained a clean dry glass-stoppered flask. She weighed the flask and stopper on an analytical balance and found the total mass to be 31.601
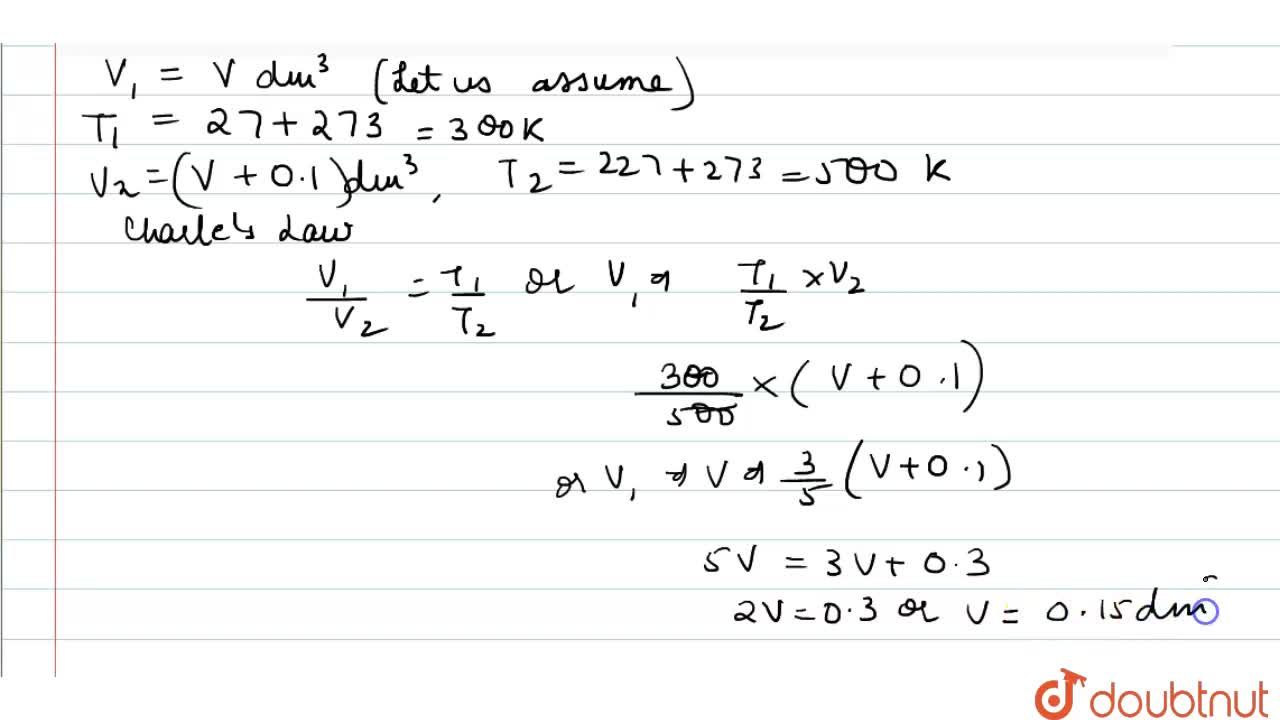
A flask was heated from 27^(@)C to 227^(@)C at constant pressure. Calculate the volume of the flask if 0.1 dm^(3) of air measured at 27^(@)C was expelled from the flask.



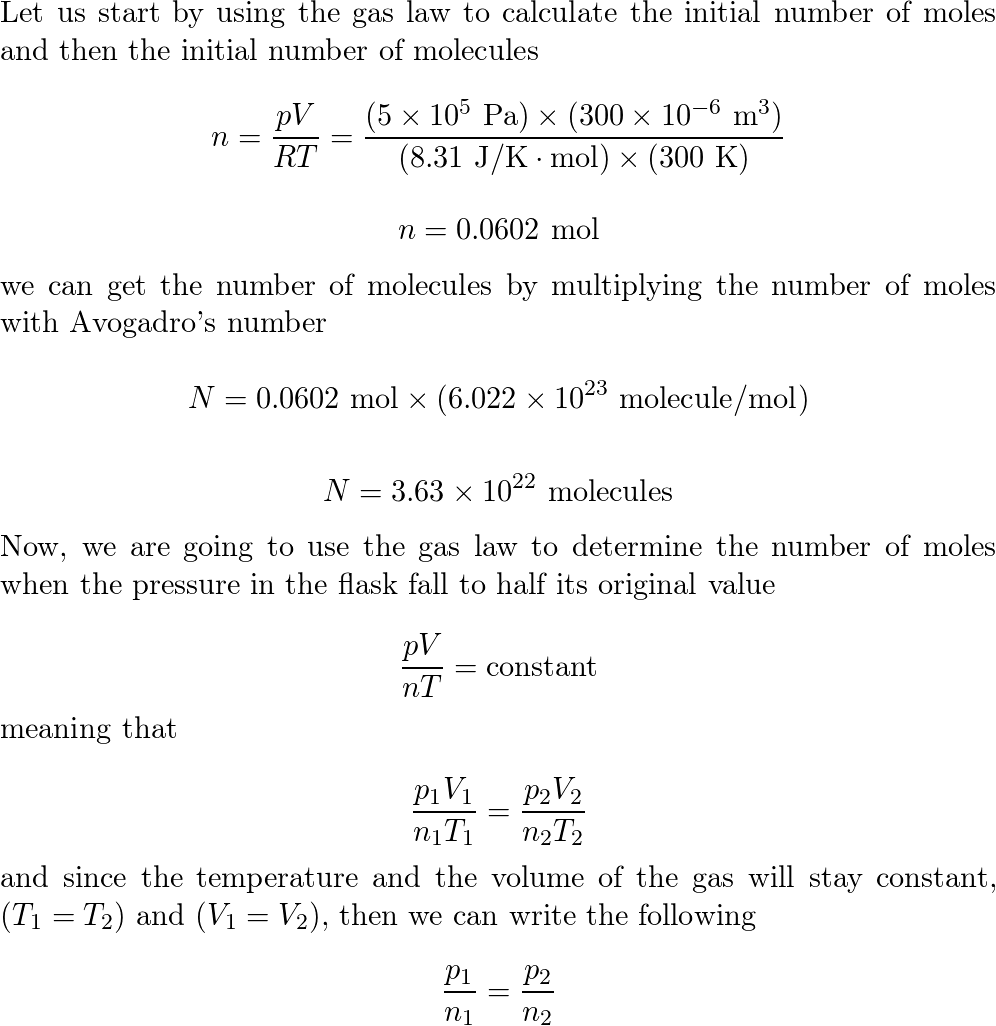
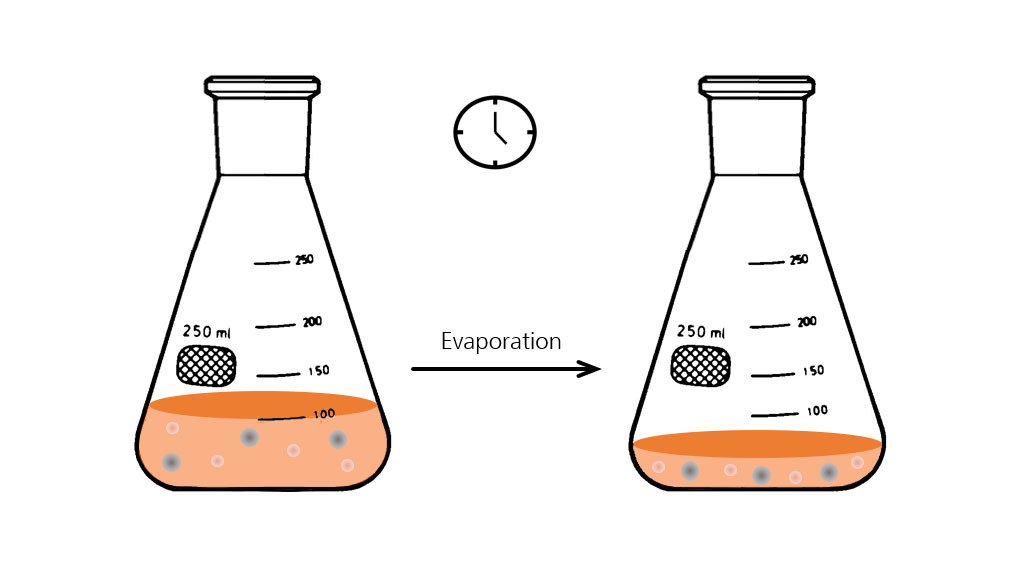
:max_bytes(150000):strip_icc()/GettyImages-493151728-c08e9b2d60bb401b8bbdd2e66de7a93b.jpg)