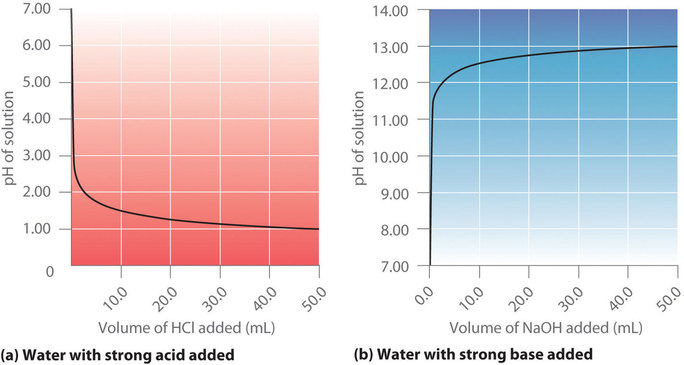
10 mL of a strong acid solution of pH = 2 are mixed with 990 mL of another acid solution of pH = 4 . The pH of the following solution will be:
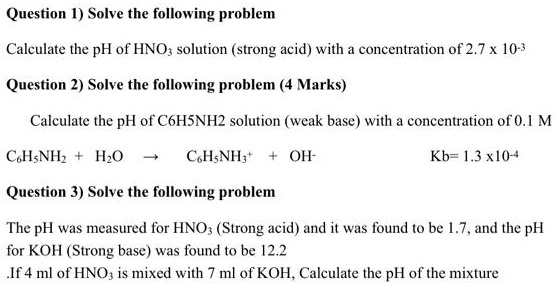
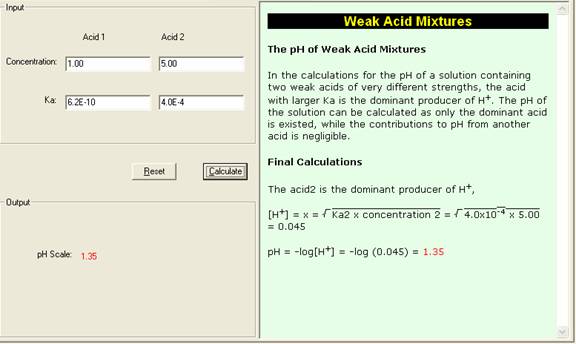
SOLVED: Question /) Solve the following problem Calculate the pH of HNO; solution (strong acid) with concentration 0f 2.7x 10-' Question 2) Solve the following problem (4 Marks) Calculate the pH of

Mixtures of Acids and Bases of PH Calculation - Study Guide | ENVE 408 | Study notes Engineering | Docsity

equilibrium - Calculation of the pH of a mixture of a strong acid and weak acid - Chemistry Stack Exchange
Determine the pH of a mixture of two weak acid (both monoprotic) solution. - Sarthaks eConnect | Largest Online Education Community

Ph calculation OF strong diprotic acid || Ph OF mixture OF strong acids or strong bases || Ph OF mixture OF strong acid and strong base || Ph OF weak monoprotic acids and bases

Calculation OF PH OF Weak Acids and Bases,PH OF Mixture OF 1)Two Strong Acids,2)Strong Acid and Strong Base,3)Strong Acid and Weak Acid,4)Two Weak Acids
![PH calculations. What is pH? pH = - log 10 [H + (aq) ] where [H + ] is the concentration of hydrogen ions in mol dm -3 to convert pH into hydrogen ion. - ppt download PH calculations. What is pH? pH = - log 10 [H + (aq) ] where [H + ] is the concentration of hydrogen ions in mol dm -3 to convert pH into hydrogen ion. - ppt download](https://images.slideplayer.com/24/7441095/slides/slide_18.jpg)